Originally posted at UTT BioPharma by: Abdul Rehman Mohammad
Cells that are old, damaged or unwanted undergo a programmed cell death. It is a biological mechanism which is (ironically) vital for survival. This process is called apoptosis, and ultimately results in the destruction of abnormal cells, preventing their accumulation. Apoptosis is essential for living multi-cellular organisms to maintain their optimal functioning, through the self destruction and regeneration of new cells.
The ‘hallmarks of cancer’ comprise of the biological capabilities acquired by human tumors during their multi-step development. Resisting cell death through inactivating apoptosis is one of the capabilities acquired by cancer cells to survive, and thus, inducing apoptosis has been established as a mechanism of anti-cancer defense.
A deeper look into apoptosis
Multi-cellular organisms are highly sophisticated, and there are regulations placed on the number of cells each tissue should contain. The number of cells in a tissue are not only controlled by the speed of which they divide, but also how fast they die. Cells which are idle in the organism undergo programmed cell death, which is defined as apoptosis. For instance, apoptosis is a key factor in organ development, as shown below.
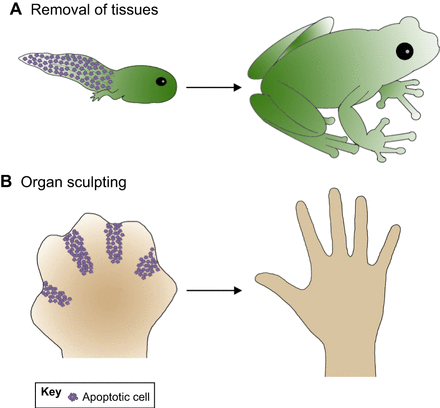
A demonstration of the importance of apoptosis in organ sculpting.
As a tadpole metamorphosizes into a frog, cells in it’s tail undergo apoptosis to remove the organ. The coordinated apoptosis of cells during embryonic development of humans leads to the formation of fingers in the hand.
Caspases are the key protein machinery responsible for apoptosis. In human cells these proteins are present as procaspases which are inactive. Once procaspases are activated they become caspases which set off a caspase cycle in turn activating more procaspases. Key protein components of the cell are broken down by the caspases, in a process called a proteolytic cascade. This includes the breakdown of the nuclear lamina and cytoskeleton, the key components which provide structural support to the cell.
The activation of apoptosis involves either an intrinsic pathway or an extrinsic pathway. In the intrinsic pathway, the release of Cytochrome C from the mitochondria binds to an adapter protein, which activates the caspases. In the extrinsic pathway, the activation of death receptors, such as Fas, activates caspases via adapter proteins.
Procaspase activation is triggered typically when the cell is stressed or damaged. Proteins of the BCL-2 family are key to promote the release of Cytochrome C from the mitochondria. Inhibitor of apoptosis proteins (IAPs) are also key proteins in the apoptosis pathway, as they bind to procaspases to prevent their activation, as well as binding to caspases to inhibit their activity. Inactivation of these complex pathways has been implicated in cancer, and apoptosis is now one of the contributors to the ‘hallmarks of cancer’.
Apoptosis and cancer
In cancer cells, there is a large reduction of apoptosis (i.e. apoptosis resistance), causing an over- accumulation of malignant cells. Various mechanisms can cause or contribute to the dysregulation of apoptosis such as impaired receptor signalling, disrupted balance of BCL-2 family of proteins, increased expression of IAPs, reduced expression of caspases as well as defects or mutations in p53 .
Treatment therapies or strategies that have been designed to restore the defective apoptotic signalling pathways towards normality have the potential to eliminate cancer cells, which depend on these defects to stay alive. Well known chemotherapy agents such as Cytarabine, Mitoxantrone, Etoposide, Paclitaxel, and Topotecan trigger the apoptotic pathway in different ways. Chemotherapy agents primarily work by inducing damage to the DNA of the cell, consequently triggering apoptosis in the cell. Unfortunately, they also cause damage to other normal cells in the body, causing severe side effects of cardiotoxicity, nephrotoxicity and neurotoxicity.
The endocannabinoid system and apoptosis
There is extensive evidence to suggest that the endocannabinoid system (being a homeostatic regulator) has anti-cancer actions. Activation of the cannabinoid receptors, CB1 and CB2, leads to the synthesis of ceramide. Ceramide triggersboth the ceramide-ERK and the p38 mitogen-activated protein kinase (p38MAPK) pathways respectively. Activation of both pathways promote apoptosis via the activation of caspases, as well as the release of cytochrome c from the mitochondria.
Activation of both receptors also causes the down regulation of gene transcription via the lowering of cyclic adenosine monophosphate (cAMP) levels and protein kinase A (PKA) activity. This down regulation or decreasing of the gene transcription is another mechanism by which apoptosis can be induced in the cell. Moreover, cells treated with endocannabinoids show increased levels of active caspase-3 and a decrease in the expression levels of Bcl-2 – emphasizing the role they play in governing apoptosis.
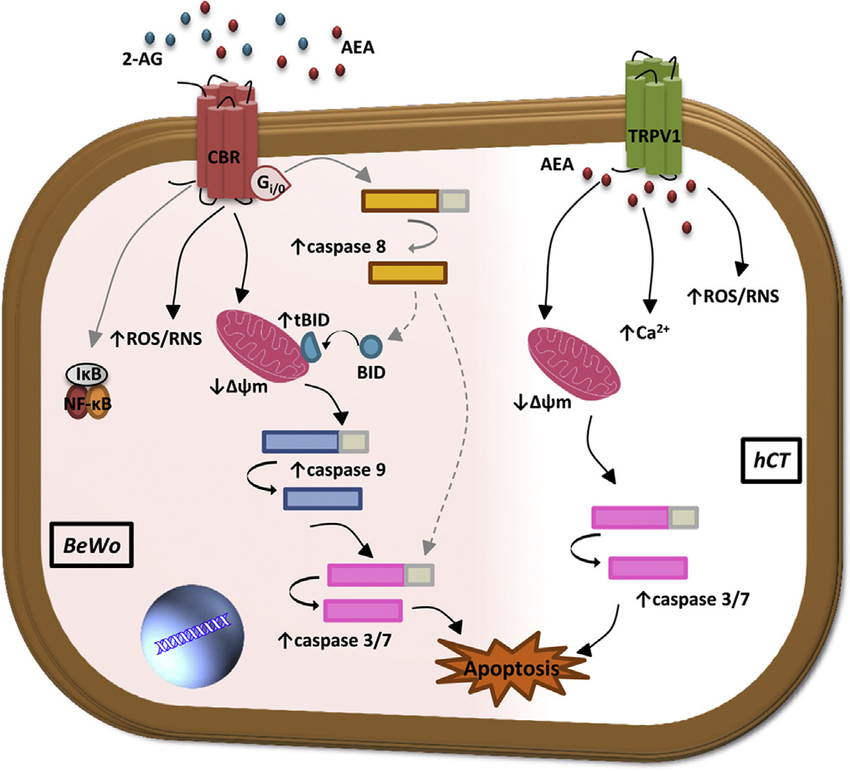
The mechanisms underlying the endocannabinoid (eCB)-induced apoptosis
Along with the cannabinoid receptors, other receptors of the endocannabinoid system, such as the Transient Receptor Potential Cation Channel V1 (TRPV1) receptors, can play a role in causing the cell to die. Activation of TRPV1 increasesintracellular hydrogen peroxide (H2O2), increase in intracellular calcium and consequently the release of cytochrome C from mitochondria. These cellular changes lead to apoptosis of the cancer cell.
Prostate cancer
Prostate cancer is characterized by abnormal cancer cells in the prostate gland that grow quicker than in a normal prostate, forming a malignant tumour. The expression levels of both the cannabinoid receptors was found to be significantly higher in prostate cancer cells, as compared to normal prostate cells. When these prostate cancer cells were treated with a CB1 and CB2 agonist, significant apoptosis occurred.
In a biopsy study (where cancer cells were extracted from prostate cancer patients and examined), high expression of the CB1 receptor was also noted. Patients with higher CB1 receptor expression also showed greater severity of prostate cancer. These patients had a significantly worse survival rate than those with lower CB1 expression levels.
It is theorized that an increase in the surface expression of CB1 receptors is a compensatory mechanism to adjust for the lower production of endocannabinoids in the extracellular environment. Taking advantage of this compensatory action of the cell could be a way to kill prostate cancers, particularly as THC caused apoptosis in a dose-dependent manner.
Lung cancer
Investigative research on the action of the cannabinoids on lung cancer is currently bare, there are many anecdotes demonstrating the anticancer activity of preparations of cannabis. The story of 54 year old Sharon Kelly, a wife and mother who was diagnosed with terminal lung cancer in December 2013, is one which demonstrates its effectiveness. After the failure of chemotherapy and other anti-cancer drugs, she turned to cannabis oil for relief. Daily cannabis oil consumption for a few months, caused her tumors to shrink significantly. A CT scan taken after seven months of cannabis oil treatment, showed there were no active cancer cells in the body.
Cannabidiol (CBD), enhanced the susceptibility of cancer cells to adhere to and subsequently be lysed by lymphokine-activated killer (LAK) cells. LAK cells trigger apoptosis in the cell through the extrinsic pathway. In another study, activation of the cannabinoid receptors halted the cell cycle at the G0/G1 phase in non-small cell lung cancer (NSCLC) cell lines. In the context of treatment option, NSCLC is a type of cancer which has resistance to many chemotherapy agents.
Breast cancer
There has been extensive research on the effects of cannabinoids on breast cancer cells, at least at the benchtop level. However there hasn’t been much progression into the clinic with a cannabinoid formulation. Breast cancer cells have shown to have high expression of the CB2 receptors, rather than the CB1 receptor. A direct correlation has been reported with the expression of CB2 receptors in ErbB2-positive tumour tissue. This is extremely relevant as ErbB2-positive breast cancer patients respond very poorly with the standard therapies which are available and relapses are extremely common in this type of breast cancer. Both THC and a CB2 agonist showed potent antitumor activity in a mouse model of ErbB2-positive breast cancer, particularly decreasing the levels of the protein Akt. The PI3K/Akt signalling pathway is often misregulated in many cancers, including breast cancer, leading to uncontrolled cell proliferation. A whole host of PI3K/Akt inhibitors are currently in clinical development.
A selective and more potent CB2 agonist, O-1663 was more effective at inducing apoptosis than THC, indicating the prominent role of the CB2 receptor as a target for the induction of apoptosis in cancer. Thus far, experimental data suggests that the CB2 receptor is a viable target for breast cancer, particularly ErbB2-positive cancer.
The bottom line
Even though all cancer cells have more or less the same functionality, to “divide and conquer”, the root cause of their malfunction is highly variable. While there might be similarities in how some cancer types manipulate the cell cycle and evade the apoptotic pathway, there are large discrepancies on how they eventually evolve to become cancer cells. The most effective treatment of cancer can only occur if there is a deeper understanding of the malfunctioning signalling pathway (s) of the tumor in question, and consequently using that information to devise the best treatment strategy for the patient. Whether cannabinoids such as THC or CBD could potentially be used to cause programmed cell death in cancer is still up for debate. Although the potential is clear. The new field of personalized medicine is highly relevant in devising cannabinoid treatment strategies for cancer. The overlap of these two areas of medicine will be essential for progress in this area.
